
Latest Issue
Just Accepted Online First List of Issues Document Types
Issue 10, 2022
EDITORIAL
2022, 40(10): 1129-1130. DOI: 10.1007/s10118-022-2863-5Published(online): 2022-09-23Full text L-PDF
RESEARCH ARTICLE
2022, 40(10): 1131-1140. DOI: 10.1007/s10118-022-2731-3Published(online): 2022-09-23Abstract Full text L-PDFAbstract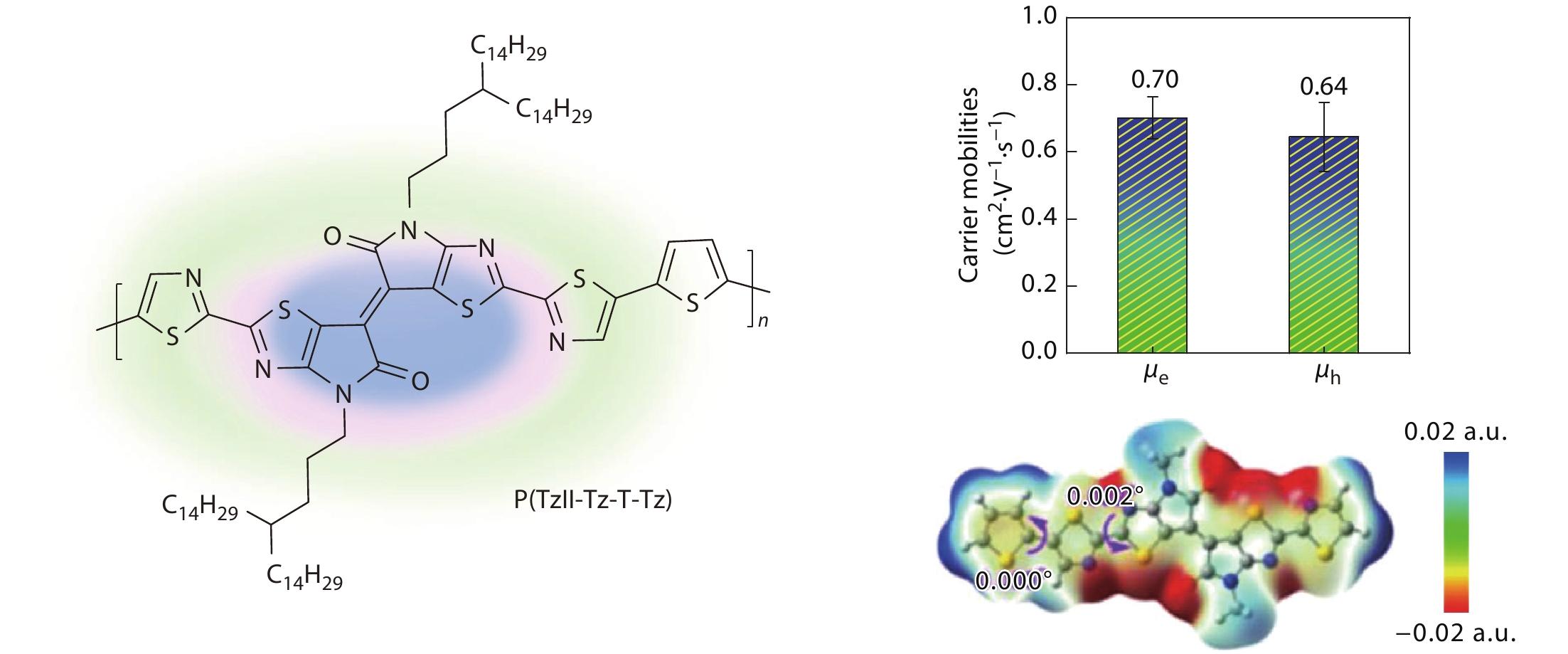
RESEARCH ARTICLE
2022, 40(10): 1141-1153. DOI: 10.1007/s10118-022-2752-yPublished(online): 2022-09-23Abstract Full text L-PDFAbstract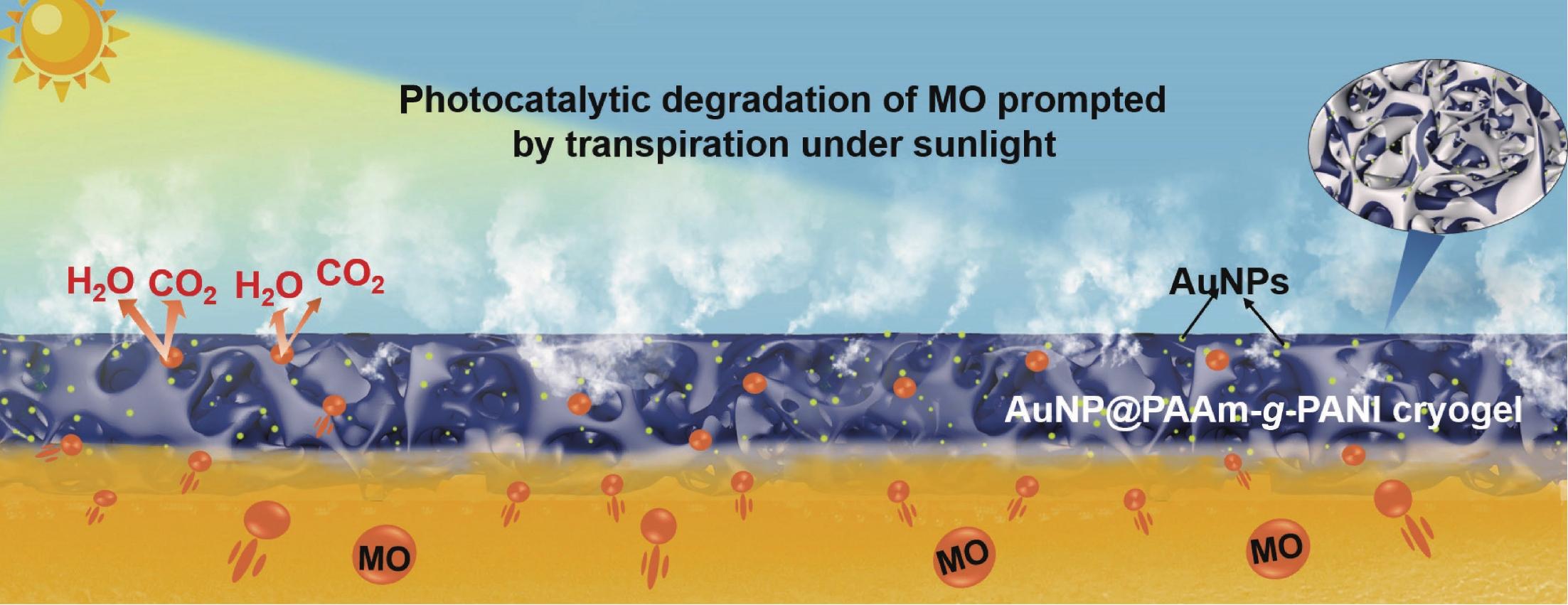
RESEARCH ARTICLE
2022, 40(10): 1154-1164. DOI: 10.1007/s10118-022-2726-0Published(online): 2022-09-23Abstract Full text L-PDFAbstract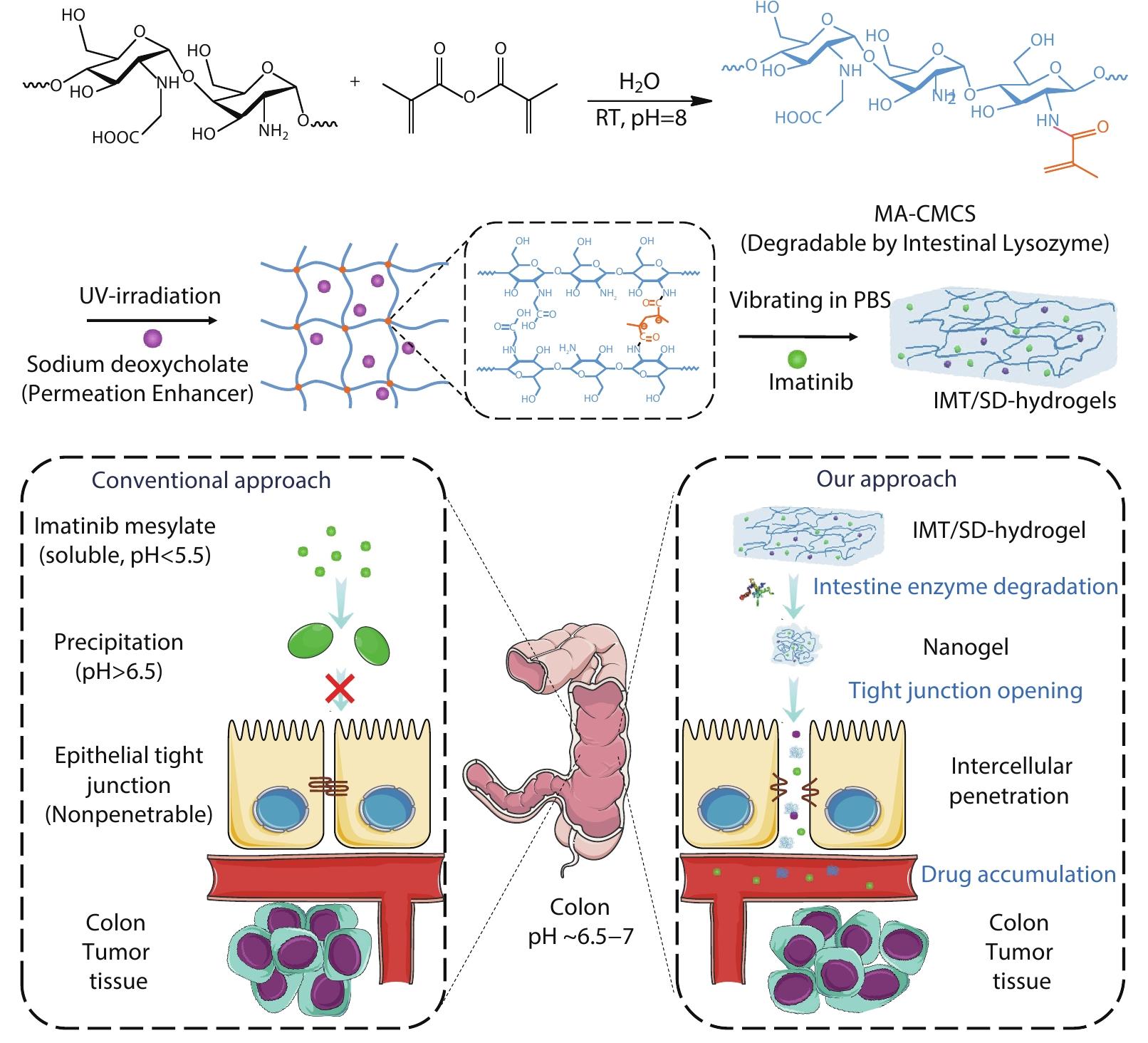
RESEARCH ARTICLE
2022, 40(10): 1165-1172. DOI: 10.1007/s10118-022-2704-6Published(online): 2022-09-23Abstract Full text L-PDFAbstract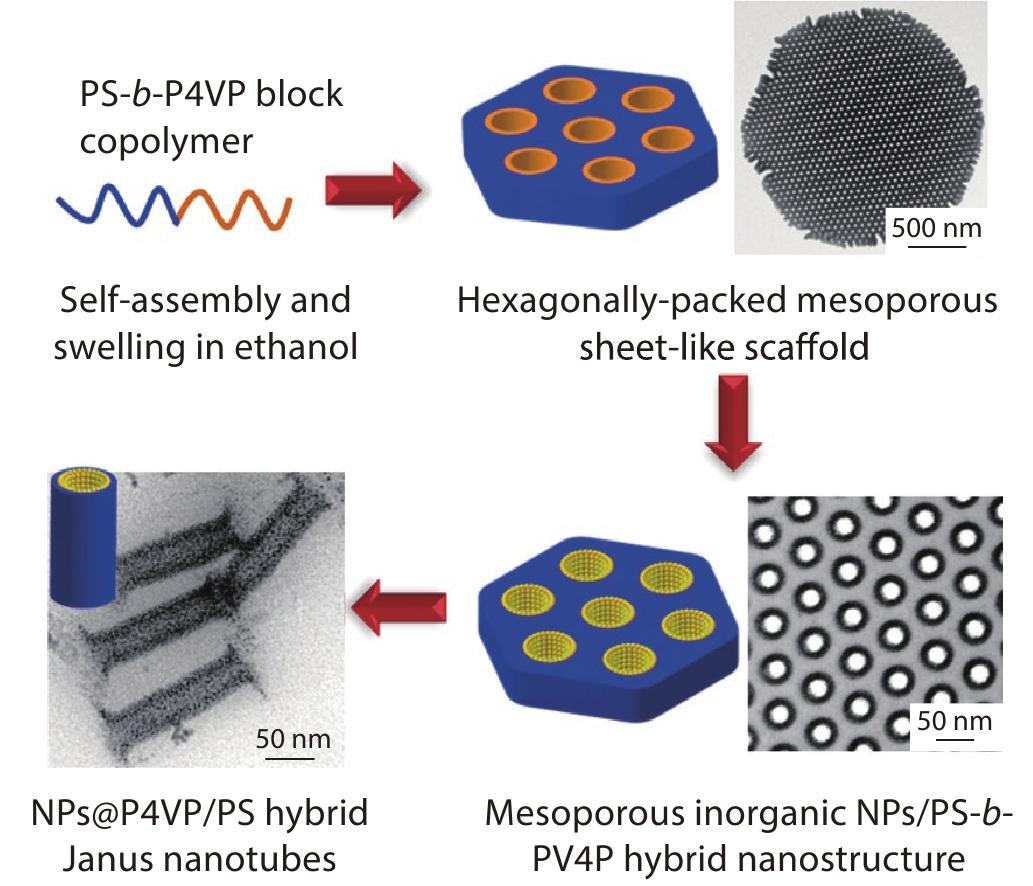
RESEARCH ARTICLE
2022, 40(10): 1173-1182. DOI: 10.1007/s10118-022-2725-1Published(online): 2022-09-23Abstract Full text L-PDFAbstract![Ring-opening Polymerization of 2-Oxabicyclo[2.2.2]octan-3-one and the Influence of Stereochemistry on the Thermal Properties of the Polyesters](https://founder-rc-product.oss-cn-zhangjiakou.aliyuncs.com/drawImage/202209/23/17/2964746570dd3155-1571-4621-a3be-db40210f8a17_m.jpg?OSSAccessKeyId=LTAI3gp3WuRqpN5T&Signature=HM7J3dHHUx5pNPSwEOKJT4M2rNk%3D&response-content-type=image%2Fjpeg)
RESEARCH ARTICLE
2022, 40(10): 1183-1192. DOI: 10.1007/s10118-022-2738-9Published(online): 2022-09-23Abstract Full text L-PDFAbstract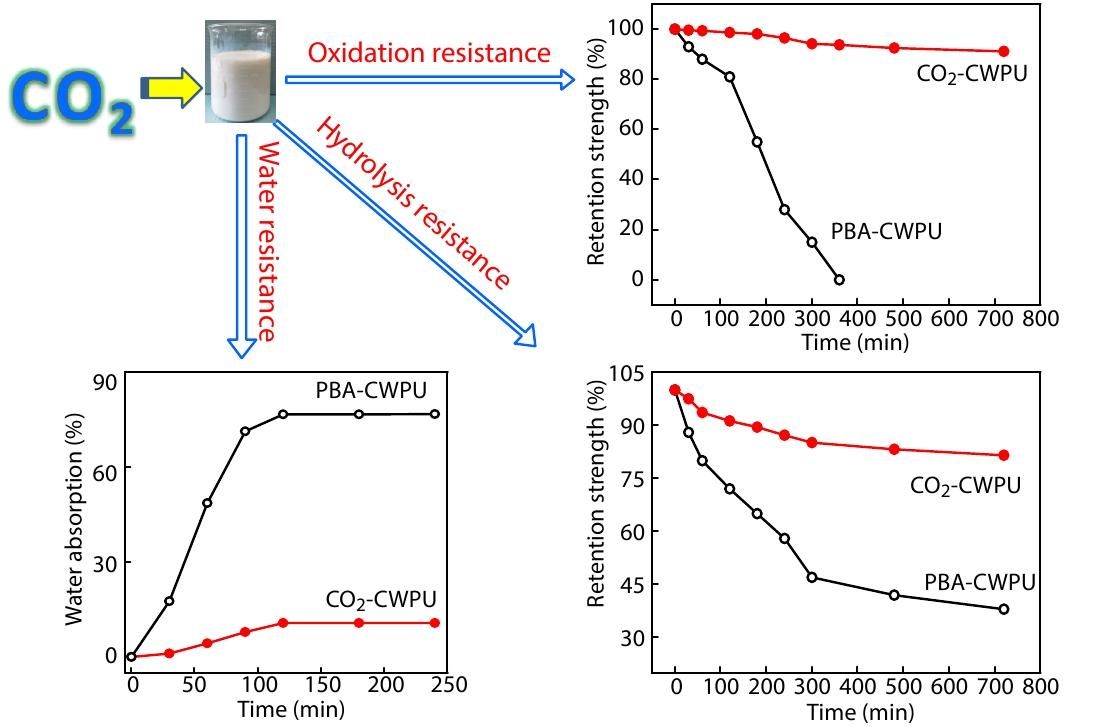
RESEARCH ARTICLE
2022, 40(10): 1193-1200. DOI: 10.1007/s10118-022-2739-8Published(online): 2022-09-23Abstract Full text L-PDFAbstract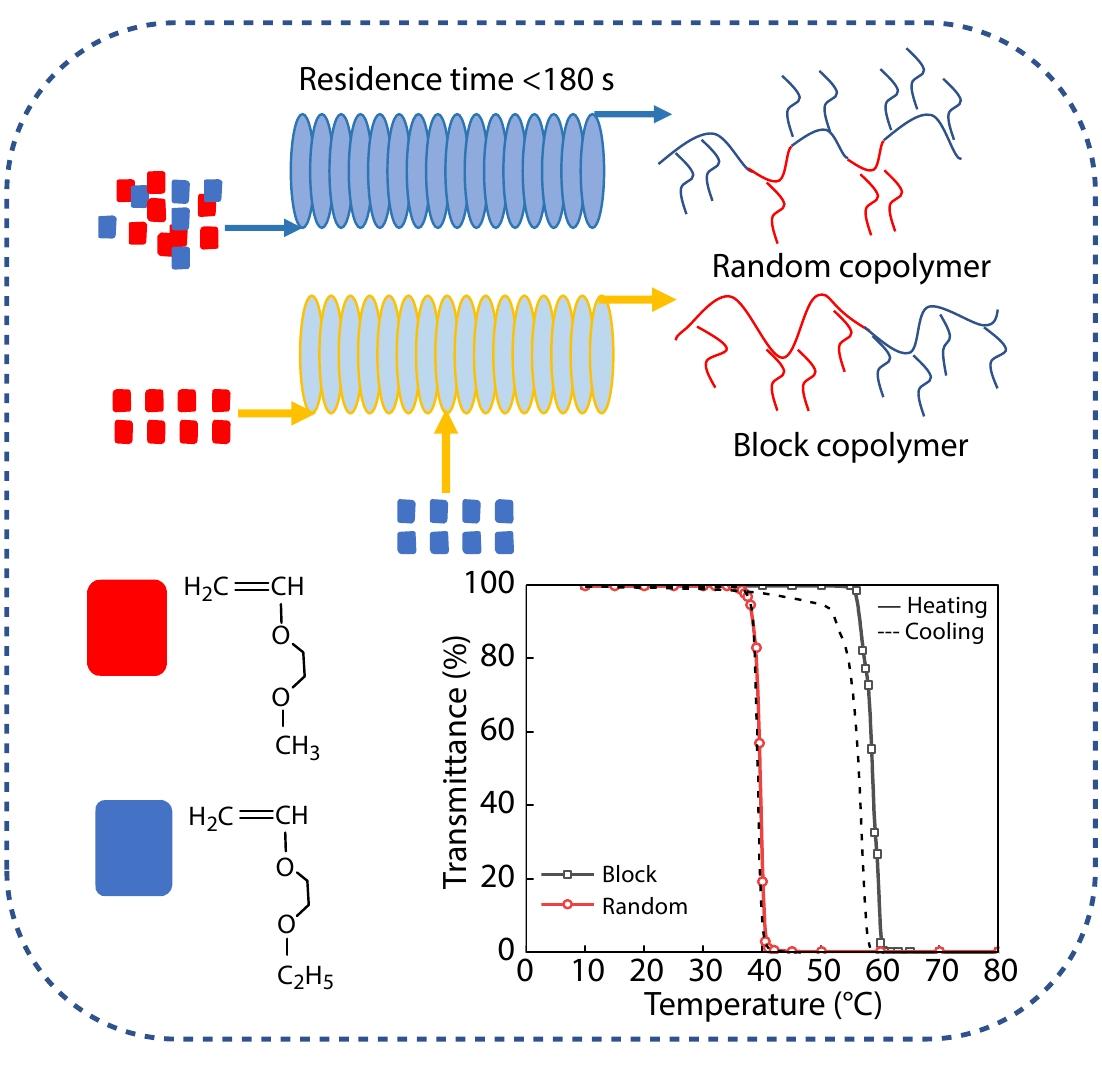
RESEARCH ARTICLE
2022, 40(10): 1201-1212. DOI: 10.1007/s10118-022-2723-3Published(online): 2022-09-23Abstract Full text L-PDFAbstract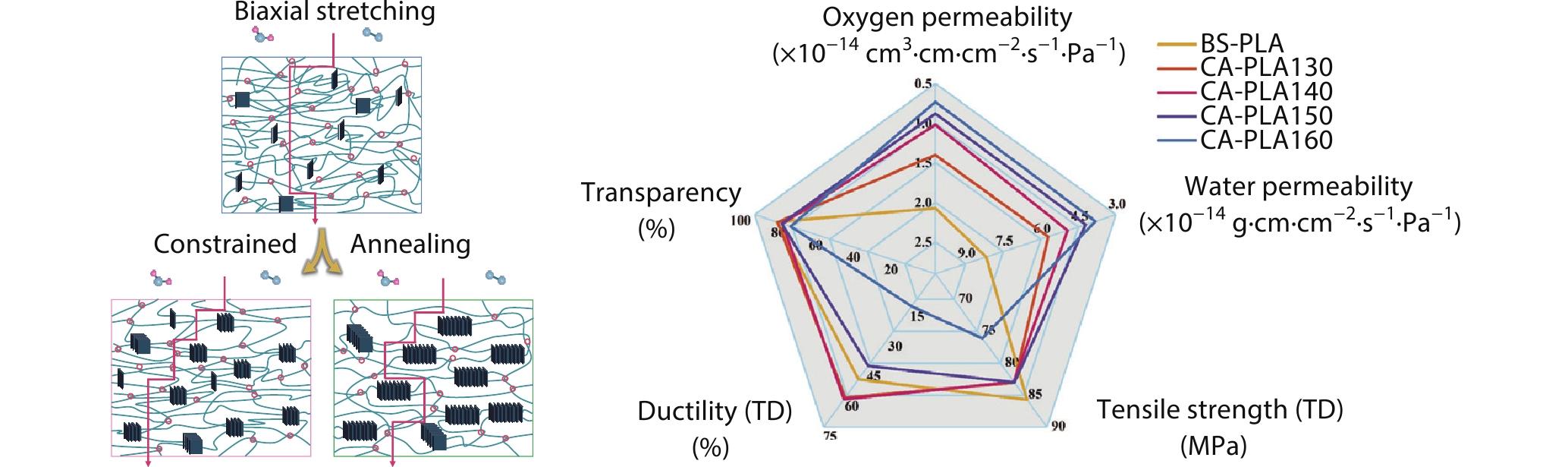
RESEARCH ARTICLE
2022, 40(10): 1213-1222. DOI: 10.1007/s10118-022-2729-xPublished(online): 2022-09-23Abstract Full text L-PDFAbstract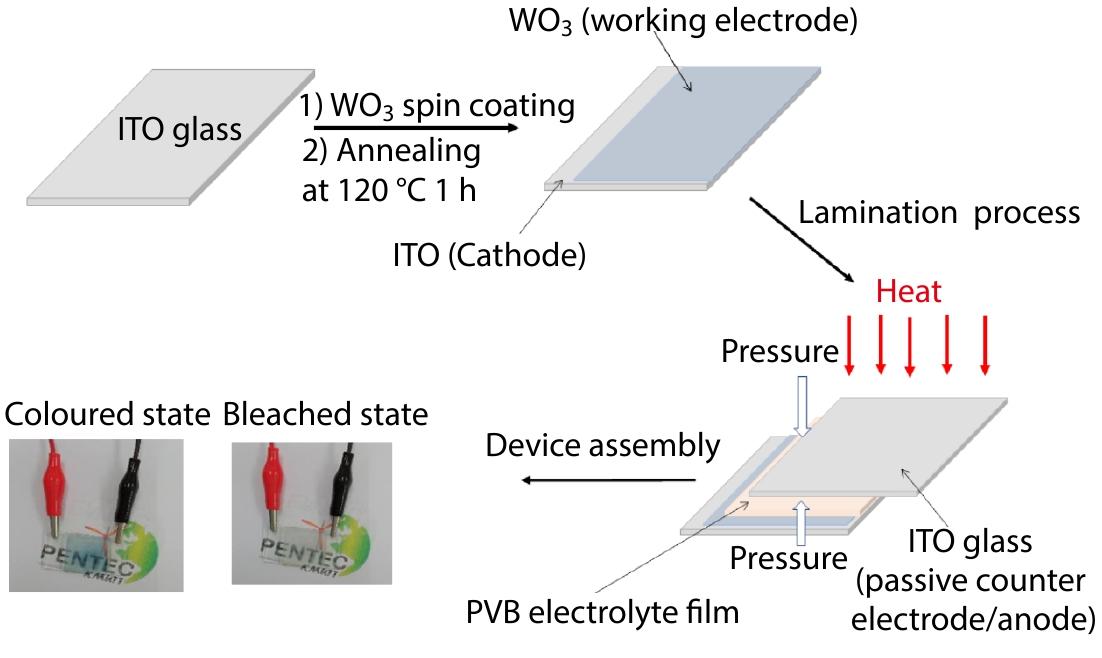
RESEARCH ARTICLE
2022, 40(10): 1223-1232. DOI: 10.1007/s10118-022-2747-8Published(online): 2022-09-23Abstract Full text L-PDFAbstract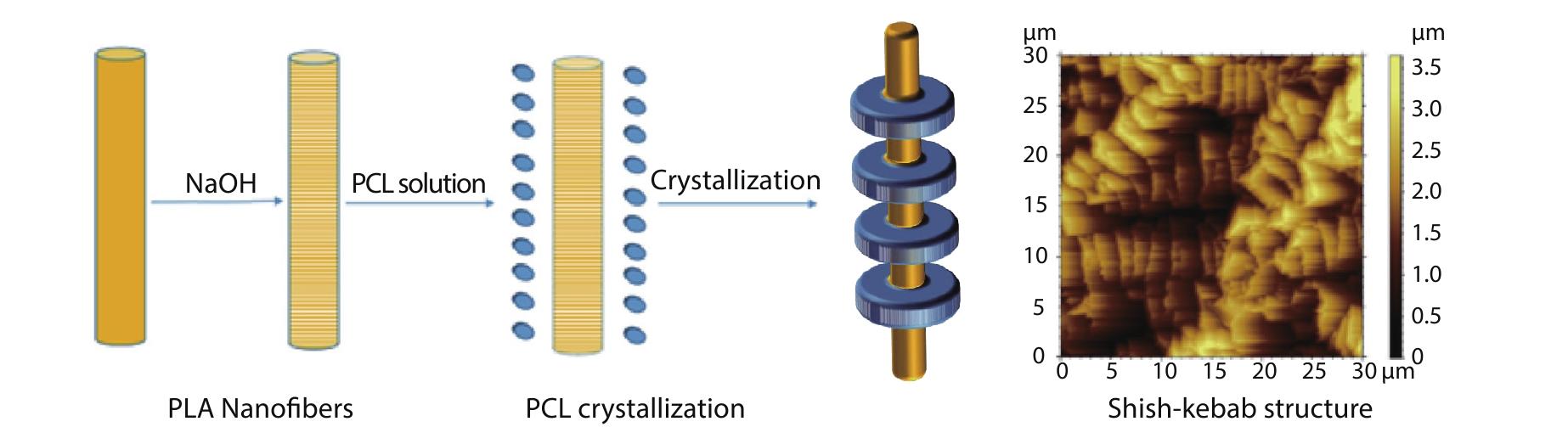
RESEARCH ARTICLE
2022, 40(10): 1233-1241. DOI: 10.1007/s10118-022-2776-3Published(online): 2022-09-23Abstract Full text L-PDFAbstract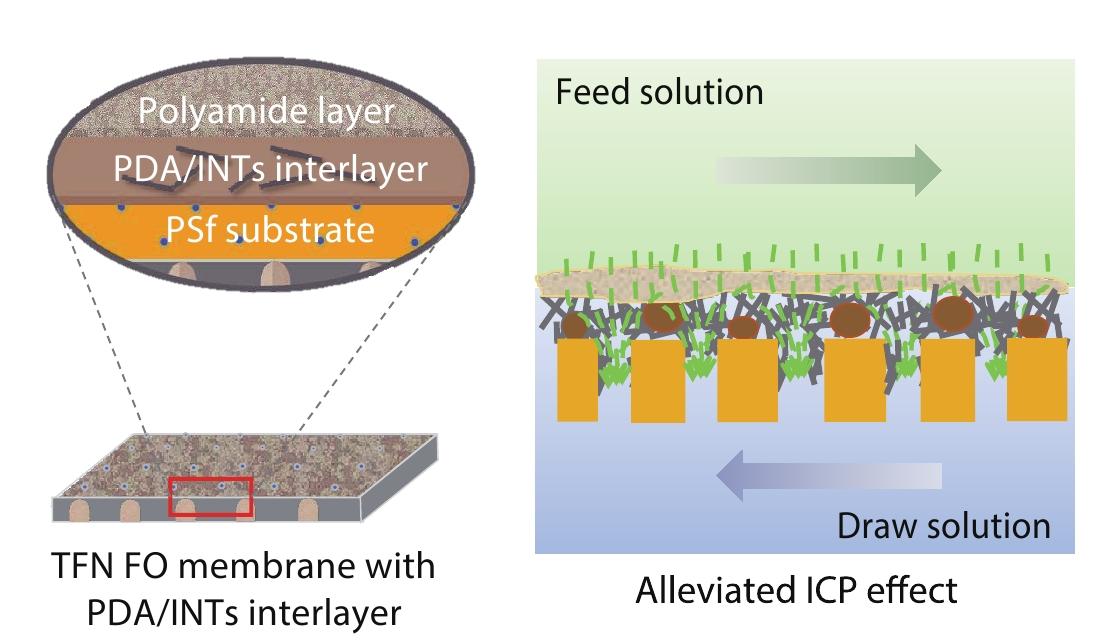
RESEARCH ARTICLE
2022, 40(10): 1242-1251. DOI: 10.1007/s10118-022-2781-6Published(online): 2022-09-23Abstract Full text L-PDFAbstract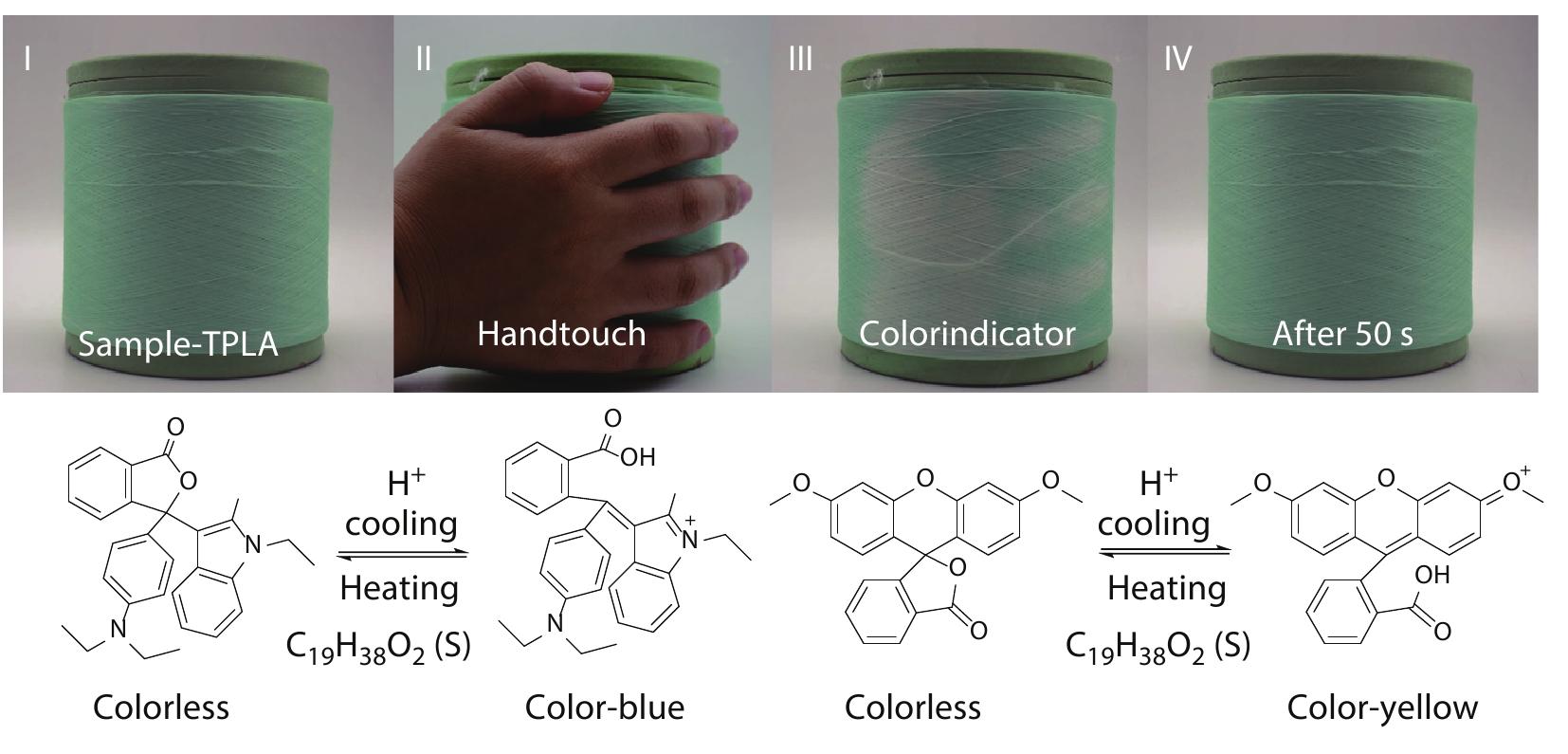
RESEARCH ARTICLE
2022, 40(10): 1252-1258. DOI: 10.1007/s10118-022-2740-2Published(online): 2022-09-23Abstract Full text L-PDFAbstract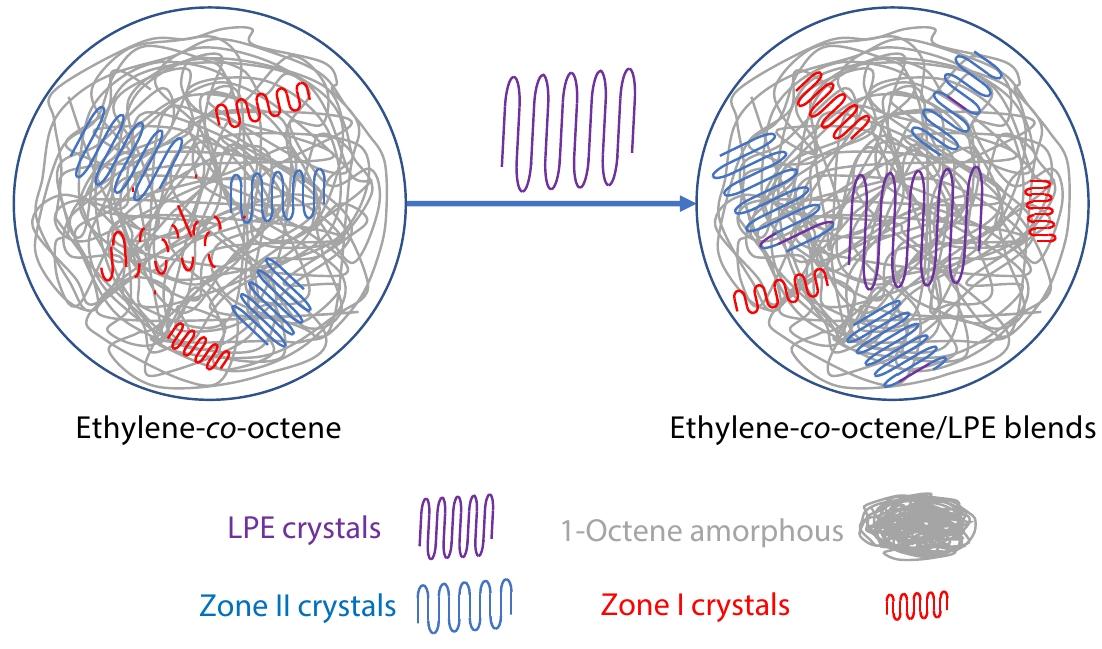
RESEARCH ARTICLE
2022, 40(10): 1259-1268. DOI: 10.1007/s10118-022-2779-0Published(online): 2022-09-23Abstract Full text L-PDFAbstract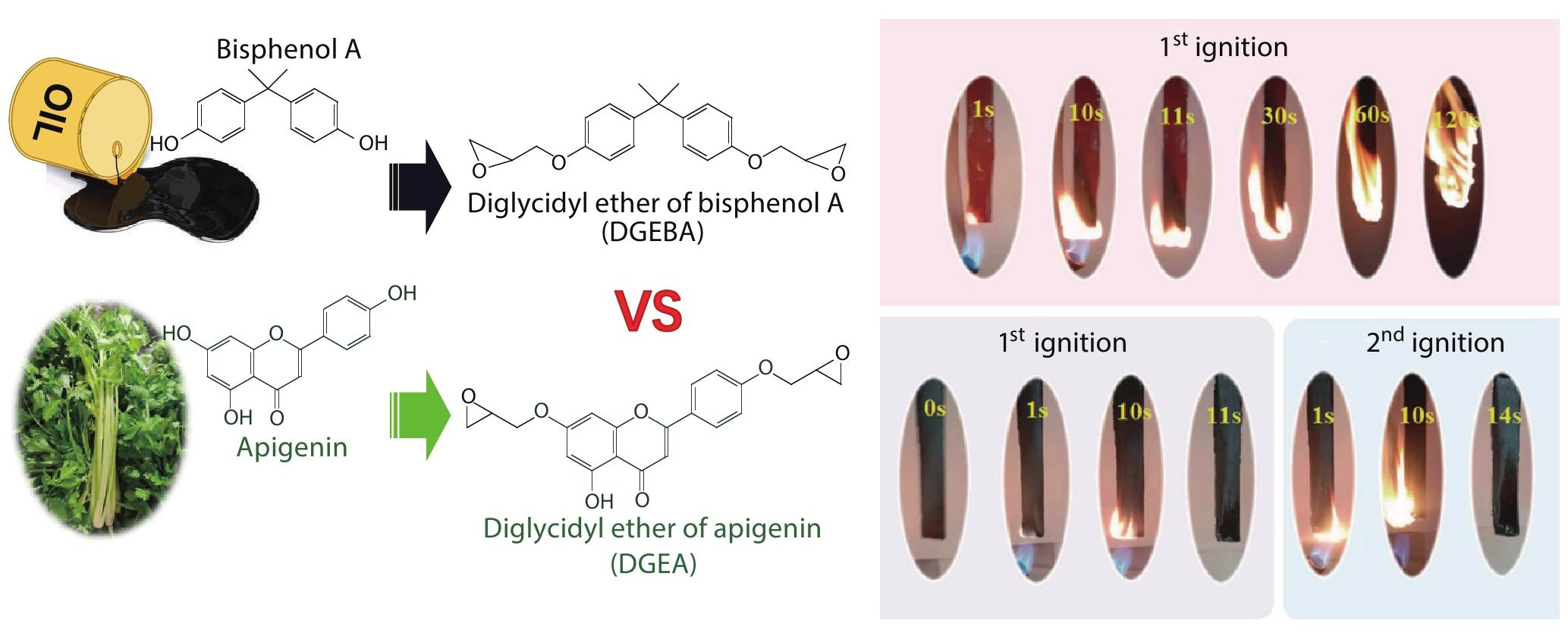
RESEARCH ARTICLE
2022, 40(10): 1269-1286. DOI: 10.1007/s10118-022-2734-0Published(online): 2022-09-23Abstract Full text L-PDFAbstract
RESEARCH ARTICLE
Palladium-assisted Metal Patterning on Polyimide Surfaces Enhanced Publication
2022, 40(10): 1287-1296. DOI: 10.1007/s10118-022-2778-1Published(online): 2022-09-23Abstract Full text L-PDFAbstract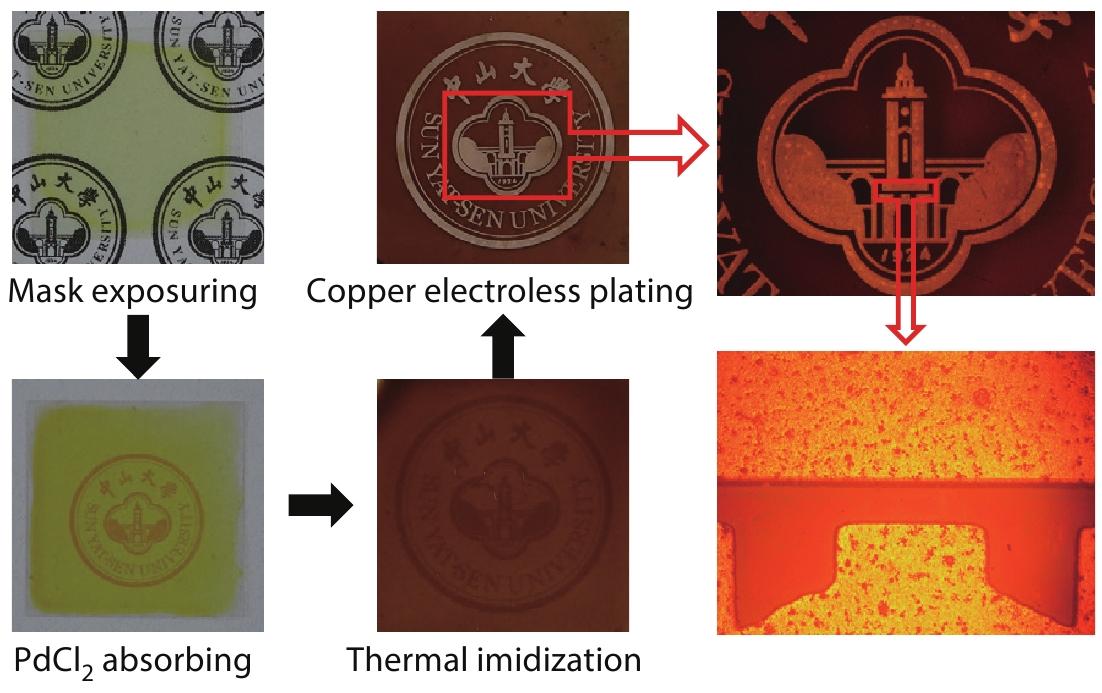
RESEARCH ARTICLE
2022, 40(10): 1297-1306. DOI: 10.1007/s10118-022-2742-0Published(online): 2022-09-23Abstract Full text L-PDFAbstract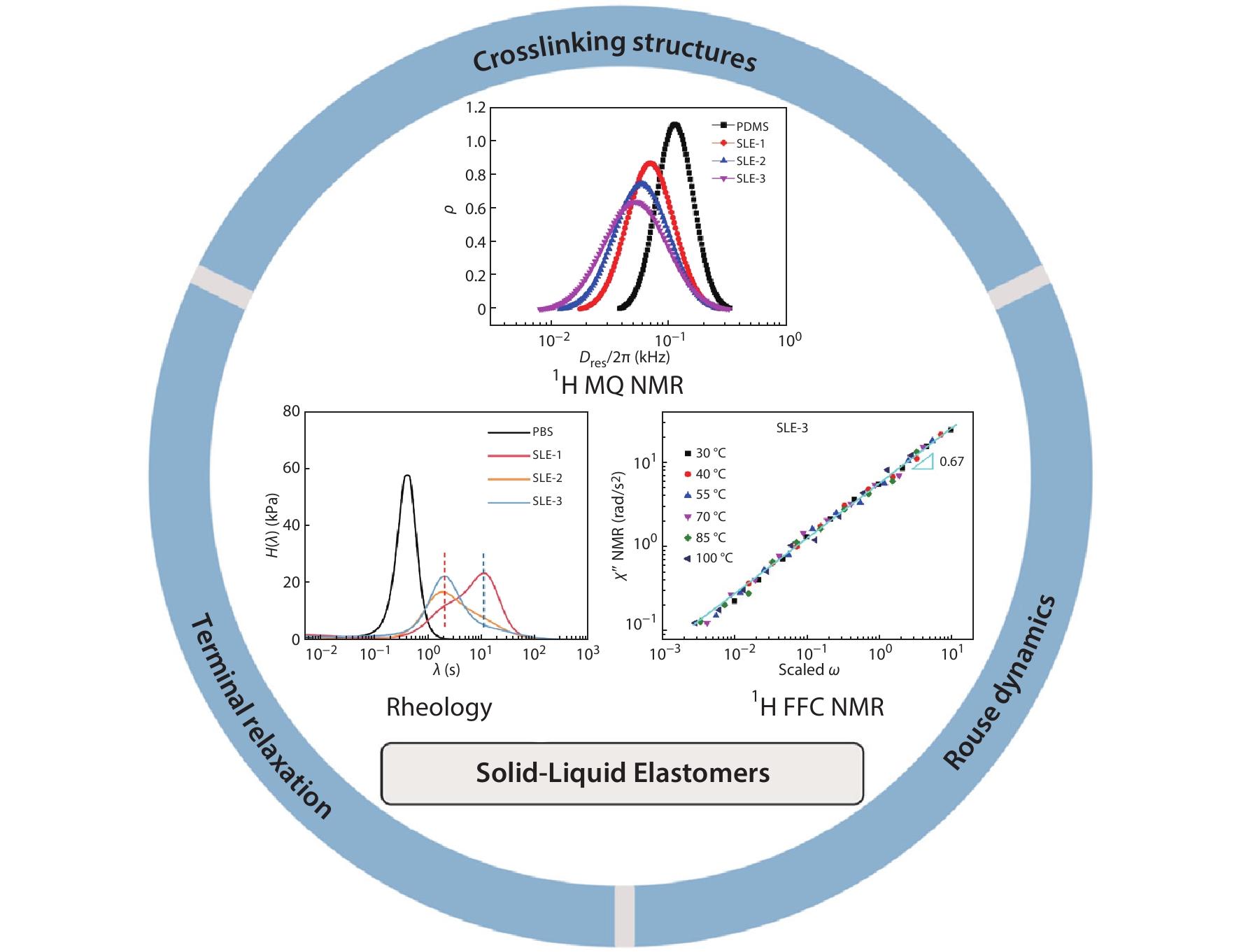
RESEARCH ARTICLE
2022, 40(10): 1307-1314. DOI: 10.1007/s10118-022-2765-6Published(online): 2022-09-23Abstract Full text L-PDFAbstract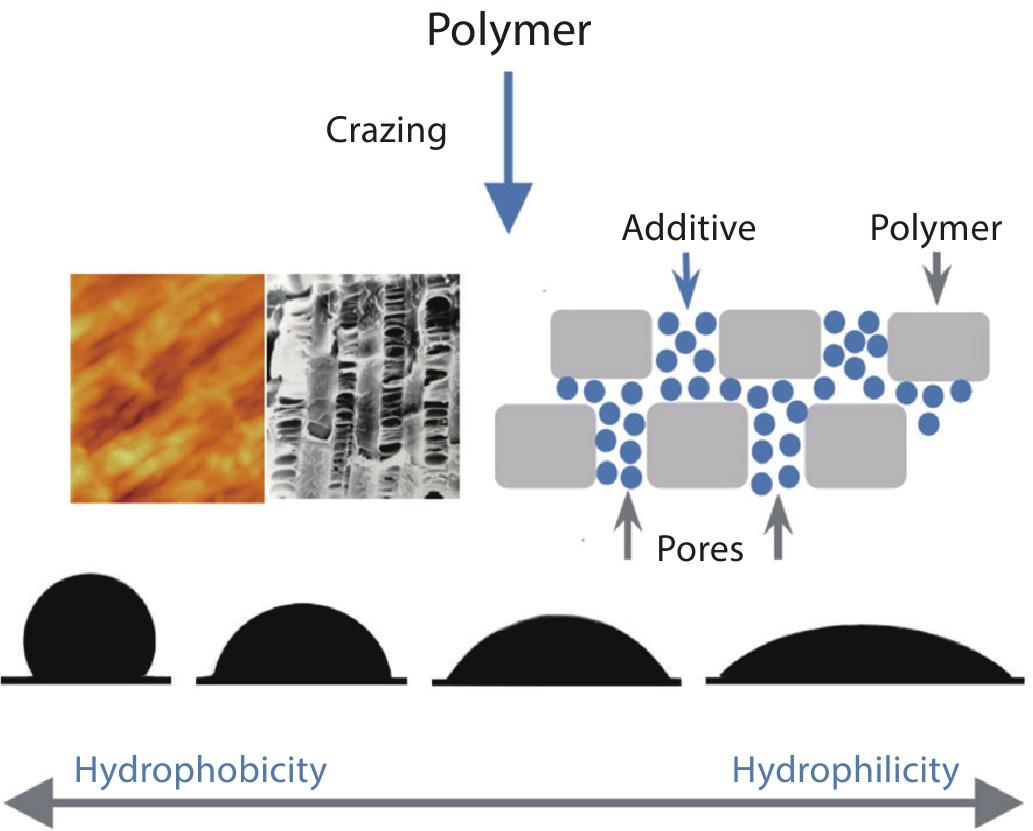
0
