Issue Ebook
cover story

Latest Issue
Just Accepted Online First List of Issues Document Types
Issue 3, 2026
REVIEW
2026, 44(3): 611-622. DOI: 10.1007/s10118-025-3548-7Published(online): 2026-02-13Abstract Full text L-PDFAbstract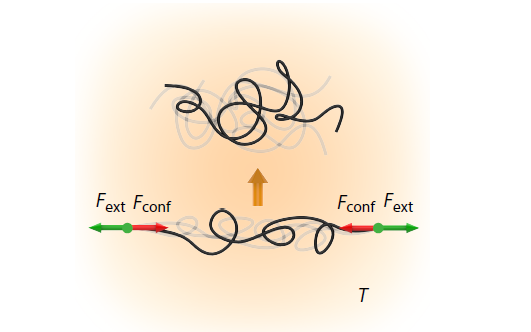
RESEARCH ARTICLE
2026, 44(3): 623-631. DOI: 10.1007/s10118-025-3542-0Published(online): 2026-02-13Abstract Full text L-PDFAbstract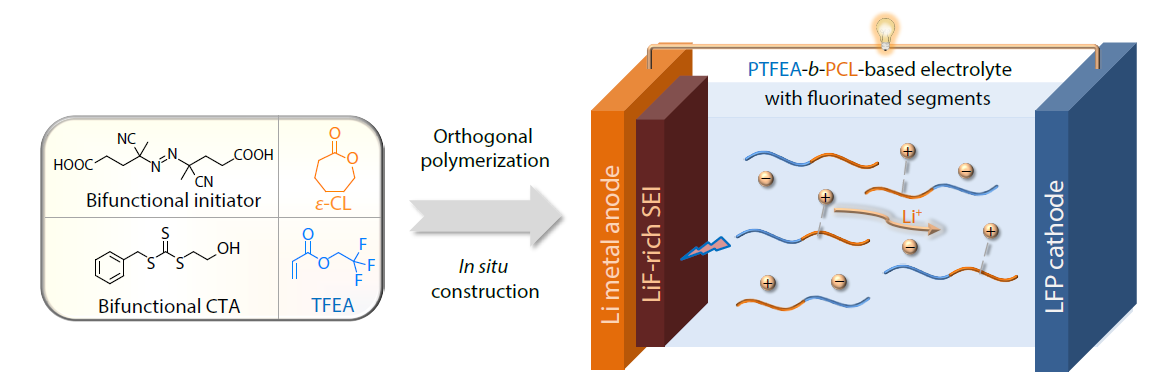
RESEARCH ARTICLE
2026, 44(3): 632-643. DOI: 10.1007/s10118-025-3543-zPublished(online): 2026-02-13Abstract Full text L-PDFAbstract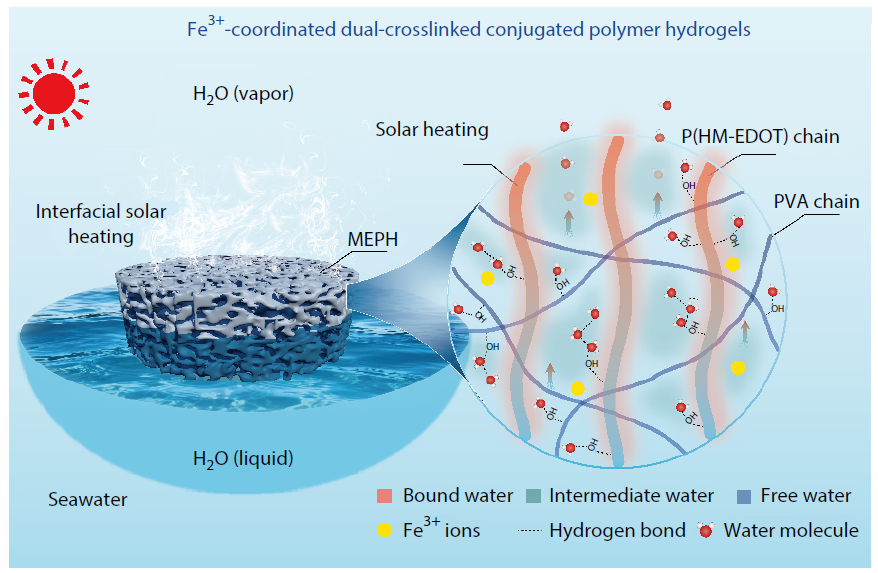
RESEARCH ARTICLE
2026, 44(3): 644-652. DOI: 10.1007/s10118-025-3534-0Published(online): 2026-02-13Abstract Full text L-PDFAbstract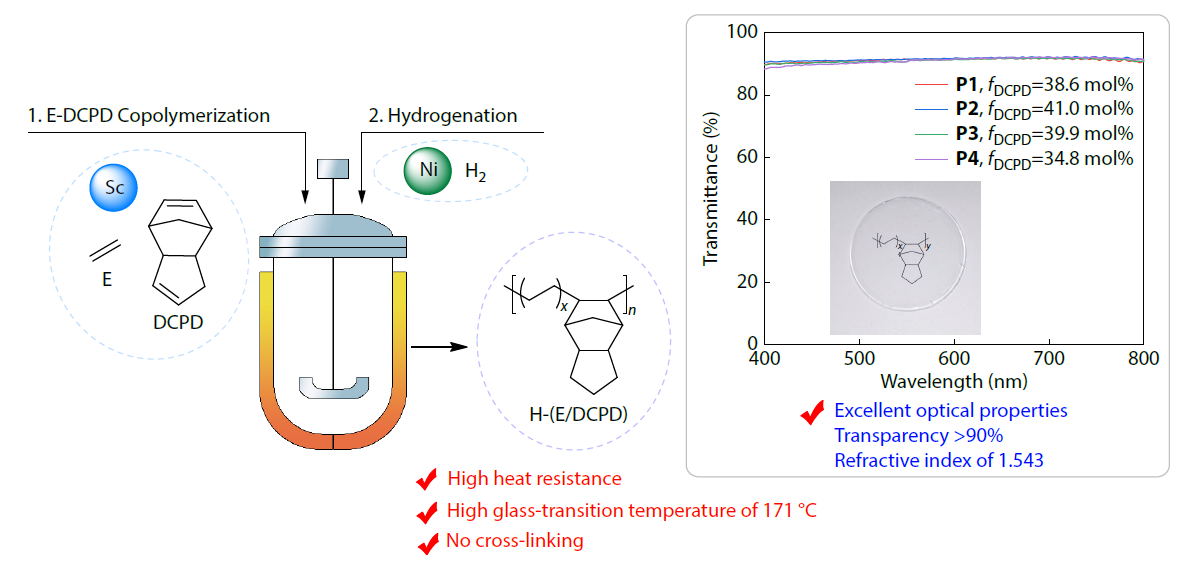
RESEARCH ARTICLE
2026, 44(3): 653-663. DOI: 10.1007/s10118-025-3535-zPublished(online): 2026-02-13Abstract Full text L-PDFAbstract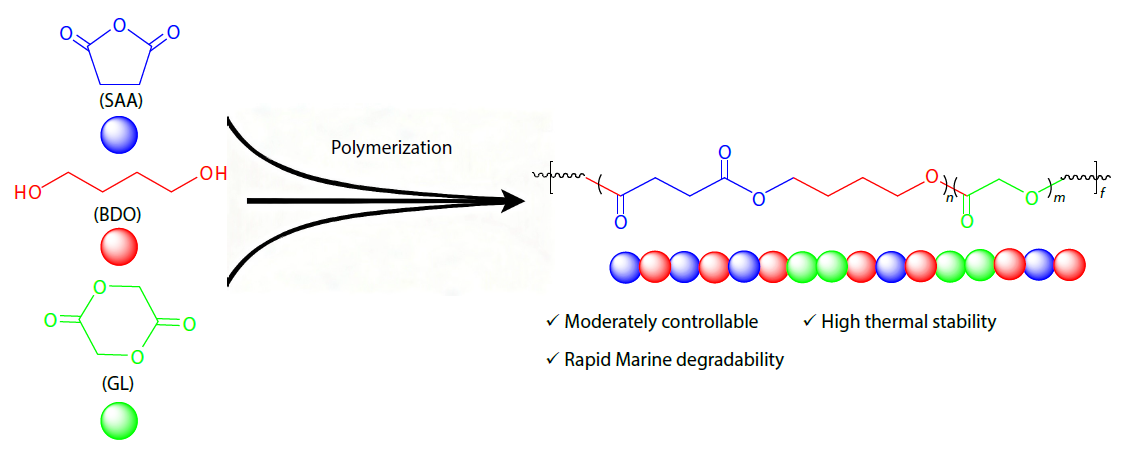
RESEARCH ARTICLE
2026, 44(3): 664-674. DOI: 10.1007/s10118-025-3540-2Published(online): 2026-02-13Abstract Full text L-PDFAbstract![Pyrazino[2,3-<italic style="font-style: italic">f</italic>][1,10]phenanthroline Derivatives as Photoredox Catalysts for Photoinduced Organocatalyzed Atom Transfer Radical Polymerization (O-ATRP) at ppm-Level Loading](https://founder-rc-product.oss-cn-zhangjiakou.aliyuncs.com/upload/2026-02-13/164J8LP11NCEM2950695.png?response-content-type=image%2Fjpeg)
RESEARCH ARTICLE
2026, 44(3): 675-687. DOI: 10.1007/s10118-025-3541-1Published(online): 2026-02-13Abstract Full text L-PDFAbstract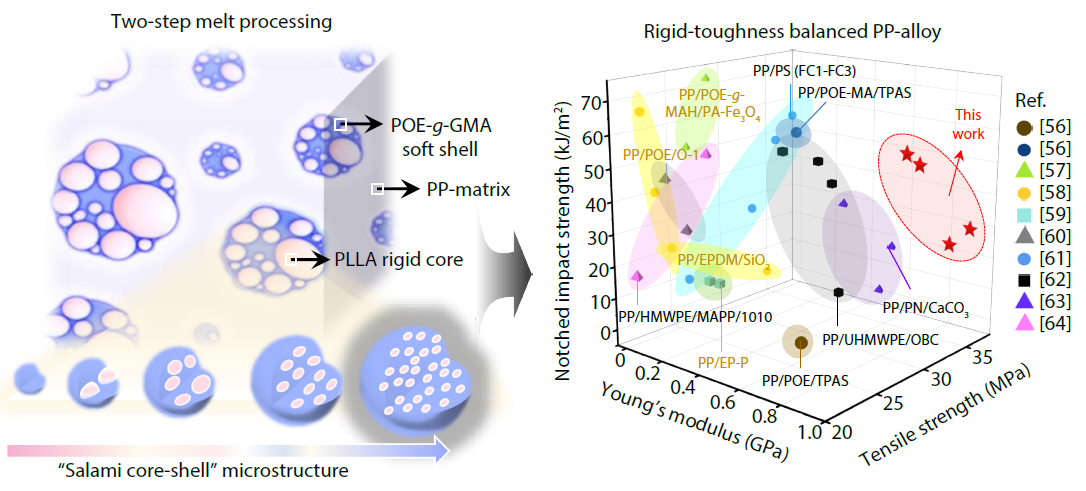
RESEARCH ARTICLE
2026, 44(3): 688-695. DOI: 10.1007/s10118-025-3537-xPublished(online): 2026-02-13Abstract Full text L-PDFAbstract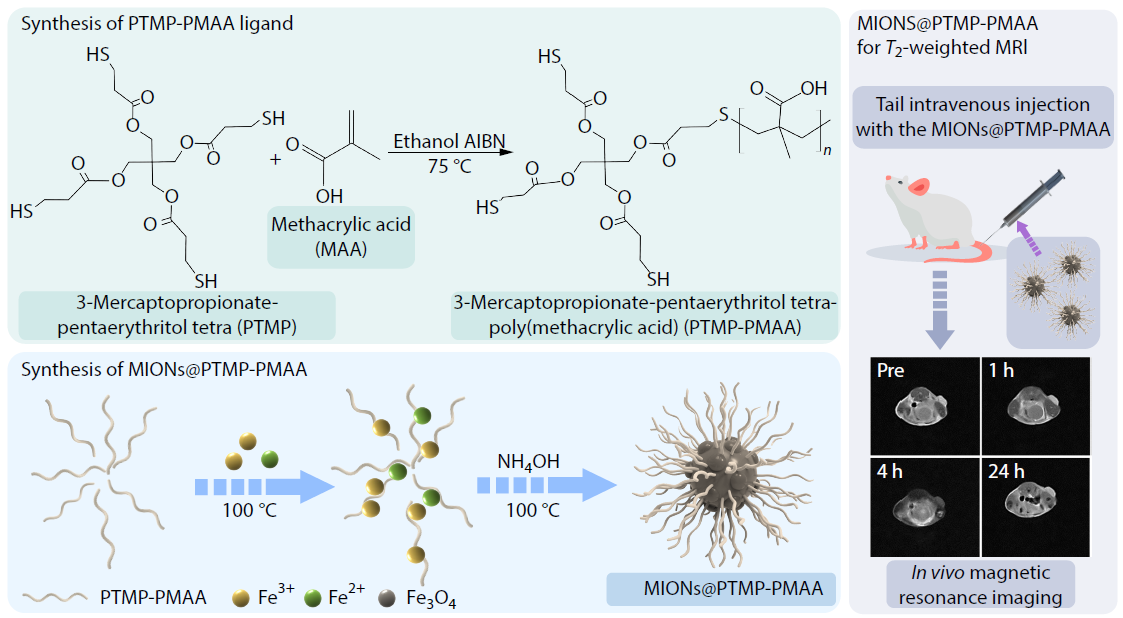
RESEARCH ARTICLE
2026, 44(3): 696-705. DOI: 10.1007/s10118-025-3523-3Published(online): 2026-02-13Abstract Full text L-PDFAbstract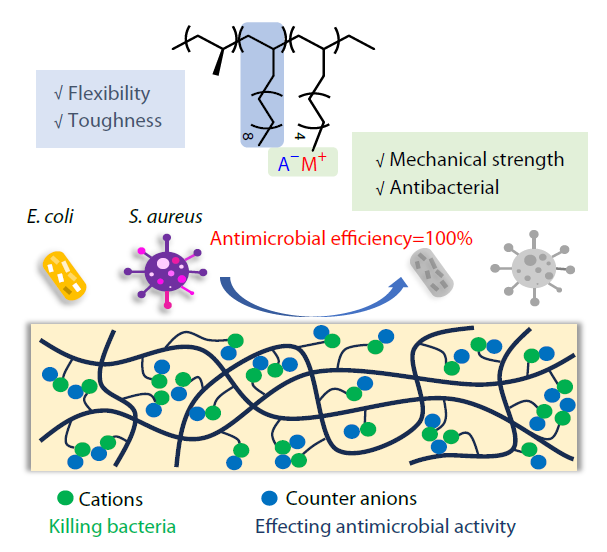
RESEARCH ARTICLE
2026, 44(3): 706-718. DOI: 10.1007/s10118-025-3549-6Published(online): 2026-02-13Abstract Full text L-PDFAbstract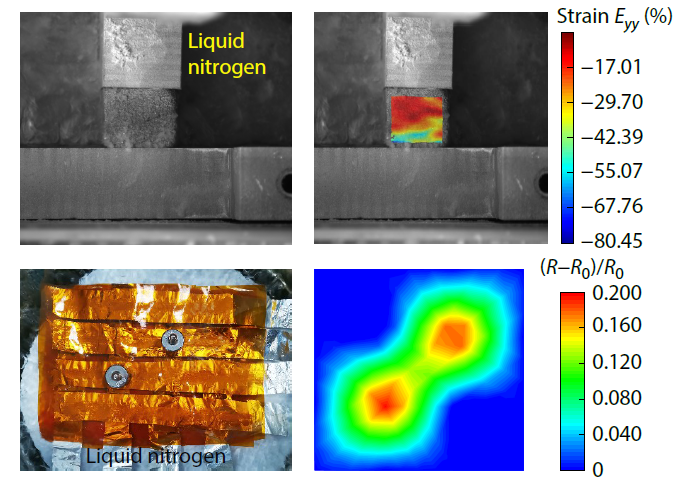
RESEARCH ARTICLE
2026, 44(3): 719-732. DOI: 10.1007/s10118-026-3553-5Published(online): 2026-02-13Abstract Full text L-PDFAbstract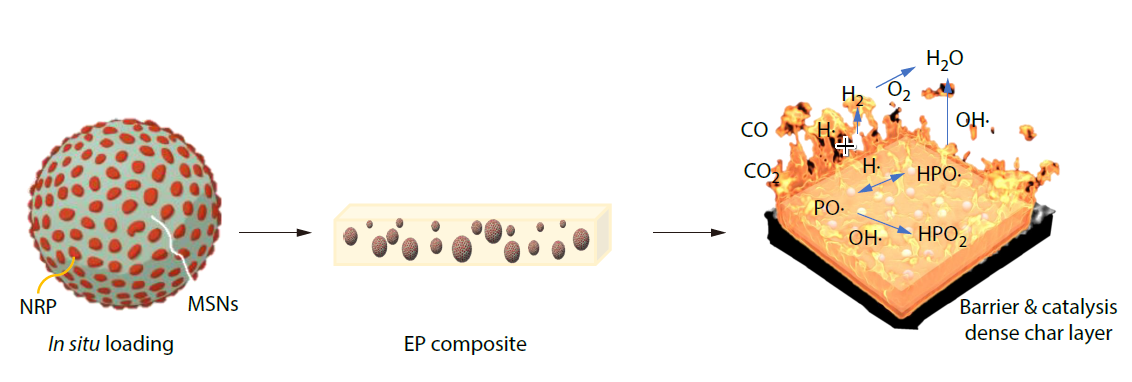
RESEARCH ARTICLE
2026, 44(3): 733-742. DOI: 10.1007/s10118-025-3530-4Published(online): 2026-02-13Abstract Full text L-PDFAbstract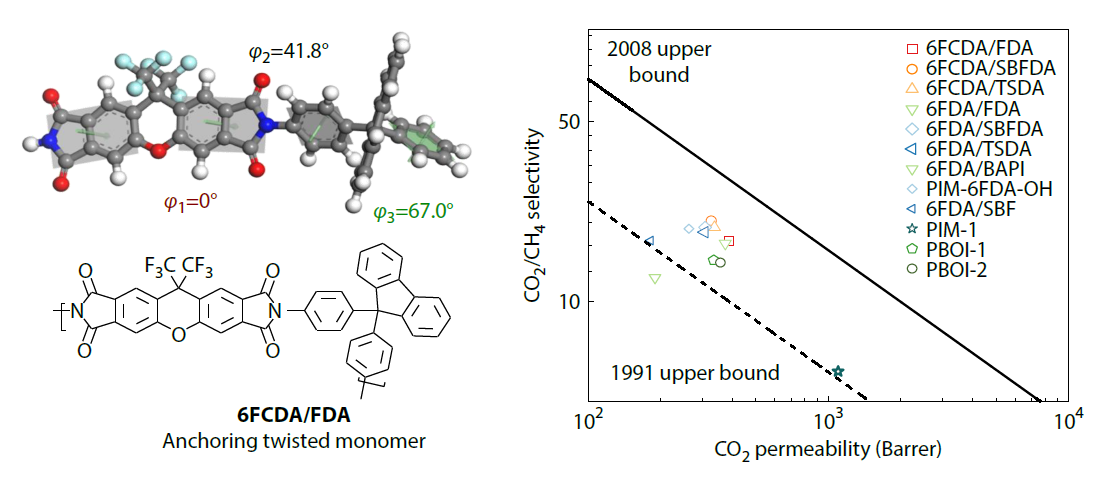
RESEARCH ARTICLE
2026, 44(3): 743-755. DOI: 10.1007/s10118-025-3532-2Published(online): 2026-02-13Abstract Full text L-PDFAbstract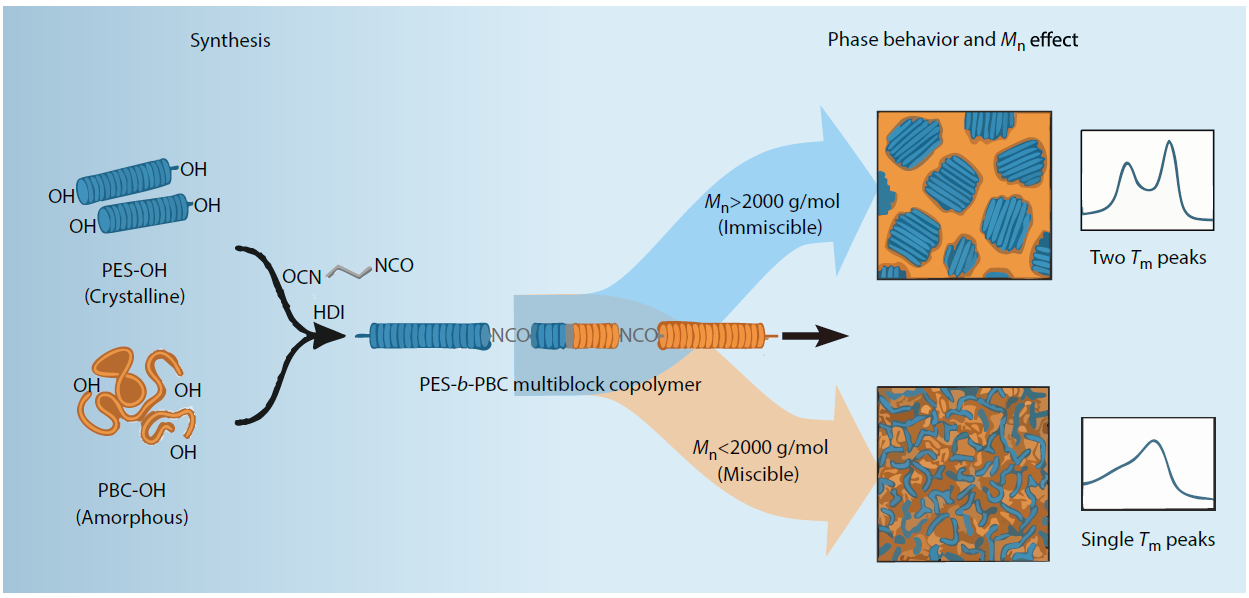
RESEARCH ARTICLE
2026, 44(3): 756-767. DOI: 10.1007/s10118-025-3529-xPublished(online): 2026-02-13Abstract Full text L-PDFAbstract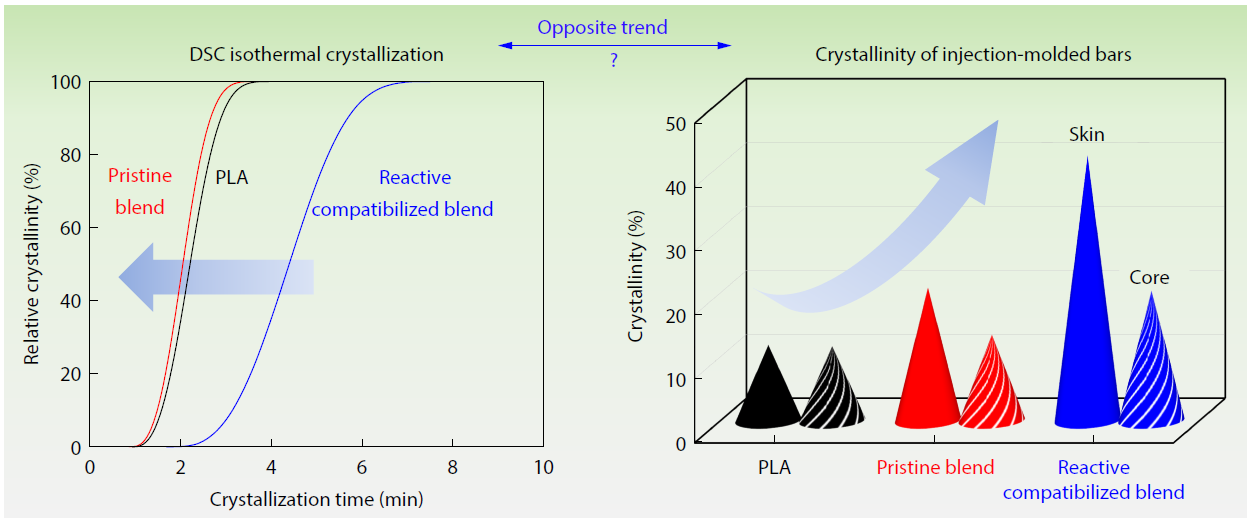
RESEARCH ARTICLE
2026, 44(3): 768-780. DOI: 10.1007/s10118-025-3547-8Published(online): 2026-02-13Abstract Full text L-PDFAbstract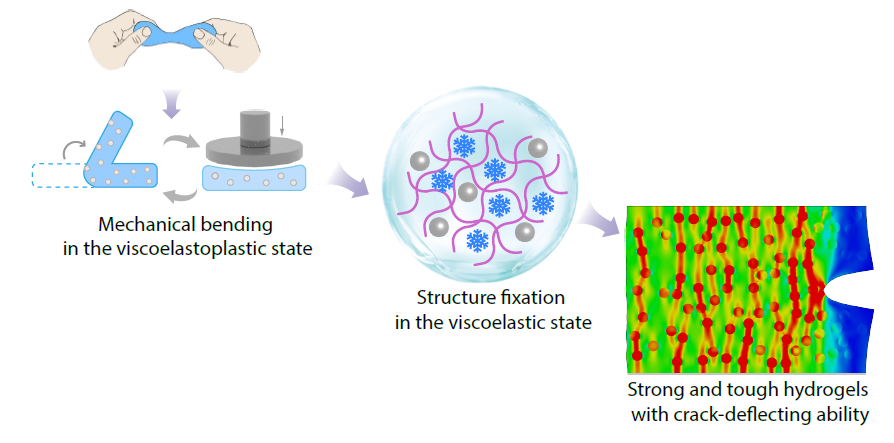
RESEARCH ARTICLE
2026, 44(3): 781-791. DOI: 10.1007/s10118-025-3539-8Published(online): 2026-02-13Abstract Full text L-PDFAbstract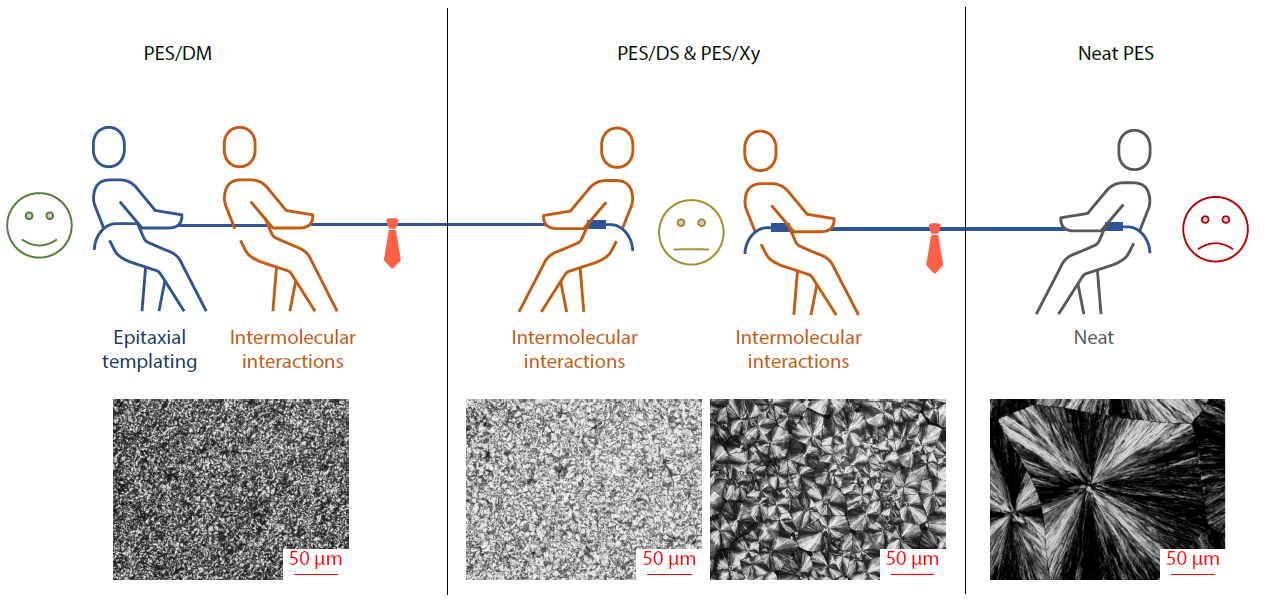
RESEARCH ARTICLE
2026, 44(3): 792-802. DOI: 10.1007/s10118-025-3527-zPublished(online): 2026-02-13Abstract Full text L-PDFAbstract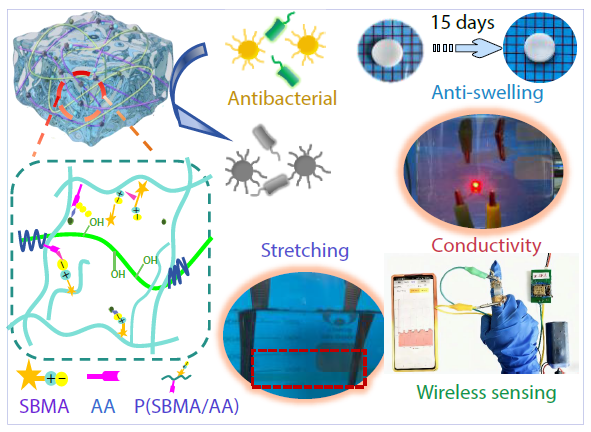
RESEARCH ARTICLE
2026, 44(3): 803-812. DOI: 10.1007/s10118-025-3520-6Published(online): 2026-02-13Abstract Full text L-PDFAbstract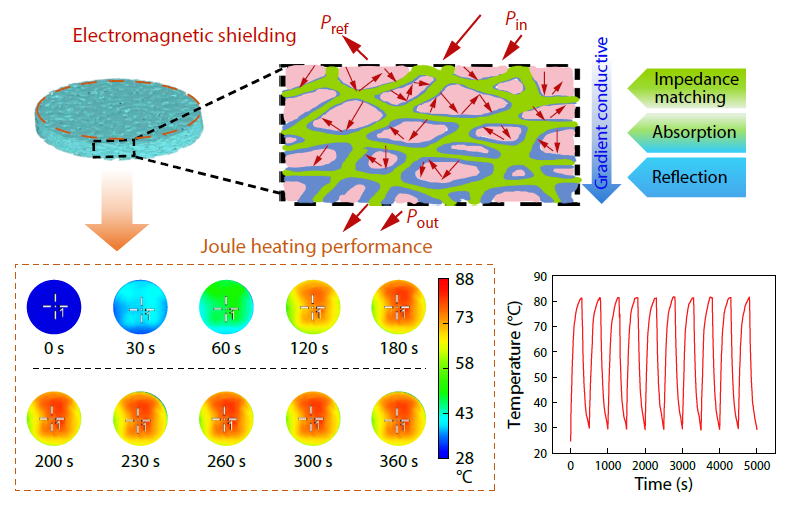
RESEARCH ARTICLE
2026, 44(3): 813-820. DOI: 10.1007/s10118-025-3524-2Published(online): 2026-02-13Abstract Full text L-PDFAbstract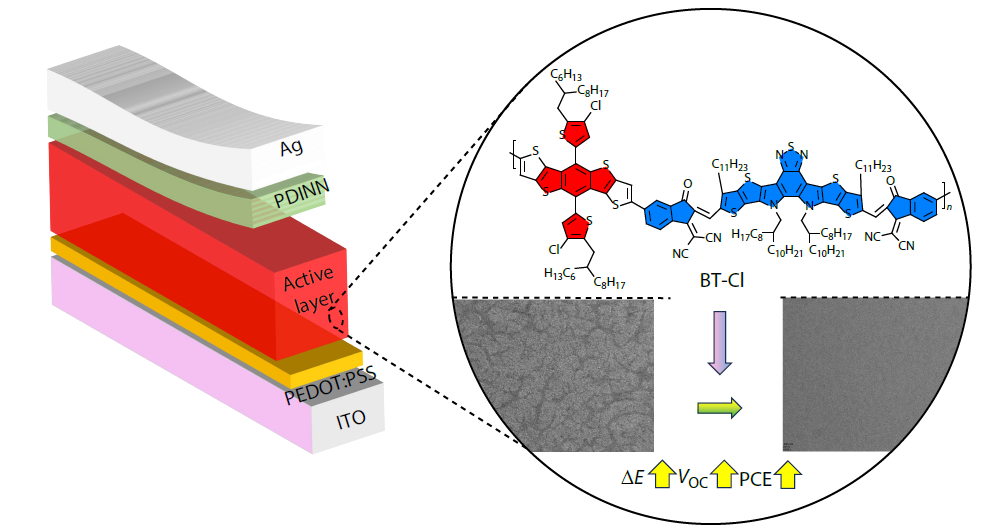
RESEARCH ARTICLE
2026, 44(3): 821-832. DOI: 10.1007/s10118-025-3521-5Published(online): 2026-02-13Abstract Full text L-PDFAbstract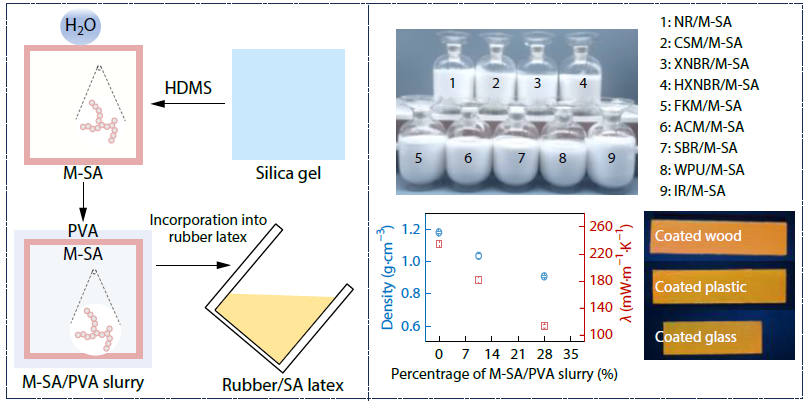
RESEARCH ARTICLE
2026, 44(3): 833-844. DOI: 10.1007/s10118-025-3544-yPublished(online): 2026-02-13Abstract Full text L-PDFAbstract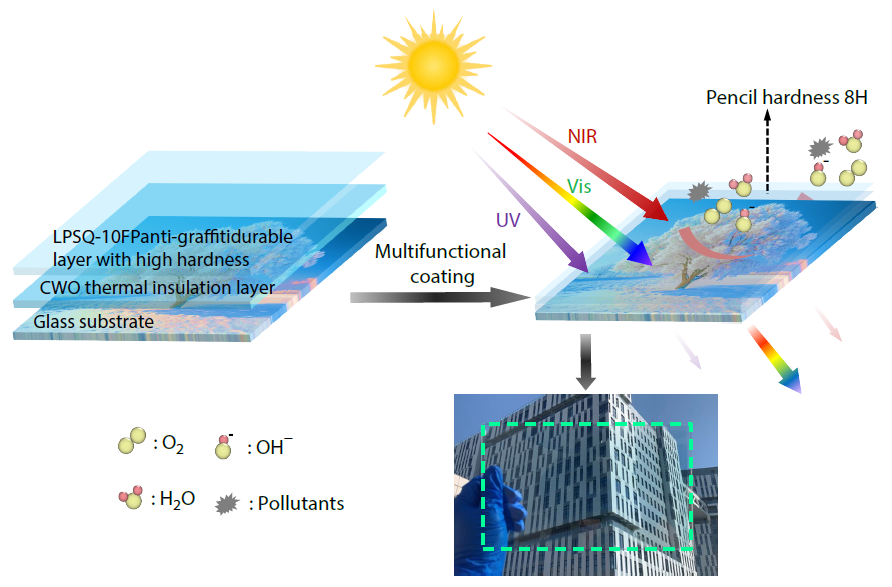
RESEARCH ARTICLE
2026, 44(3): 845-856. DOI: 10.1007/s10118-025-3507-3Published(online): 2026-02-13Abstract Full text L-PDFAbstract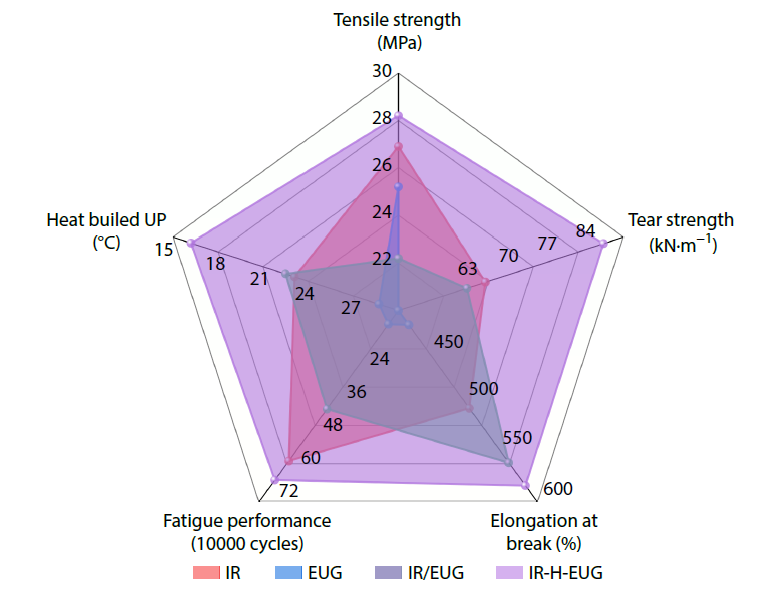
RESEARCH ARTICLE
2026, 44(3): 857-868. DOI: 10.1007/s10118-025-3546-9Published(online): 2026-02-13Abstract Full text L-PDFAbstract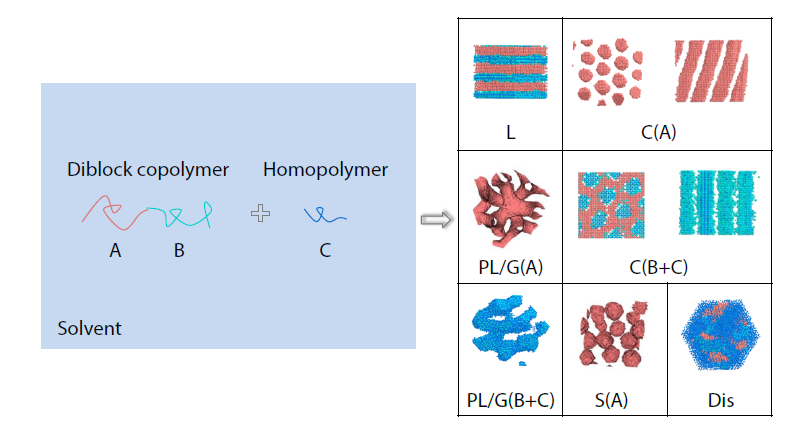
RESEARCH ARTICLE
2026, 44(3): 869-881. DOI: 10.1007/s10118-025-3538-9Published(online): 2026-02-13Abstract Full text L-PDFAbstract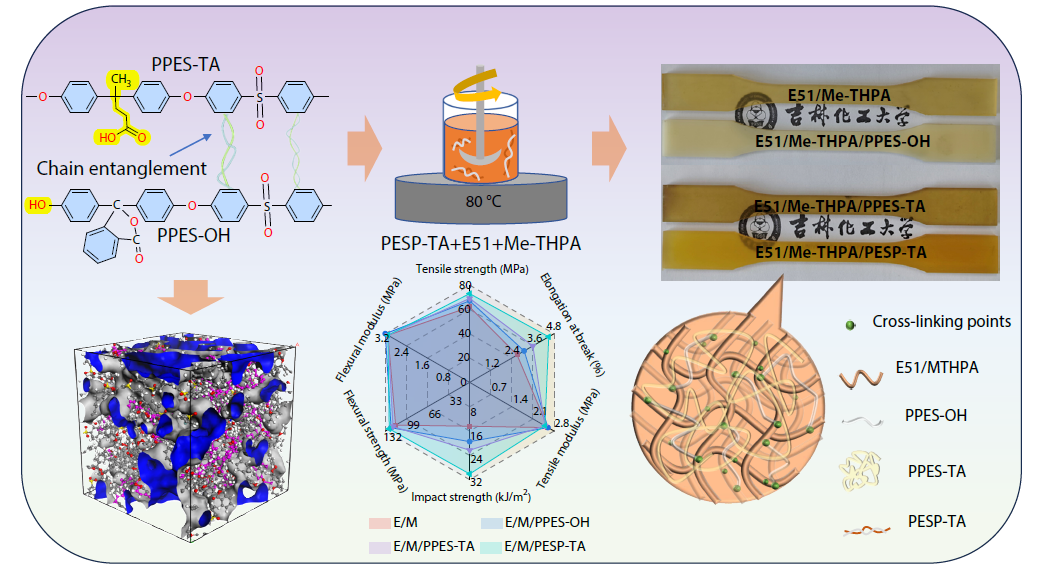
RESEARCH ARTICLE
2026, 44(3): 882-893. DOI: 10.1007/s10118-025-3533-1Published(online): 2026-02-13Abstract Full text L-PDFAbstract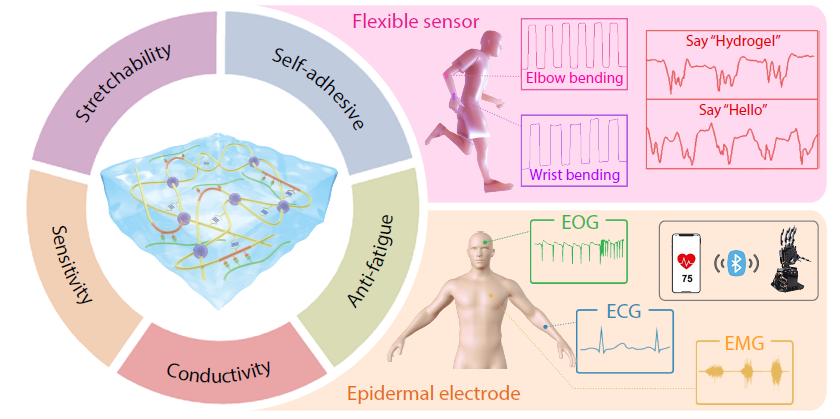
RESEARCH ARTICLE
2026, 44(3): 894-904. DOI: 10.1007/s10118-025-3528-yPublished(online): 2026-02-13Abstract Full text L-PDFAbstract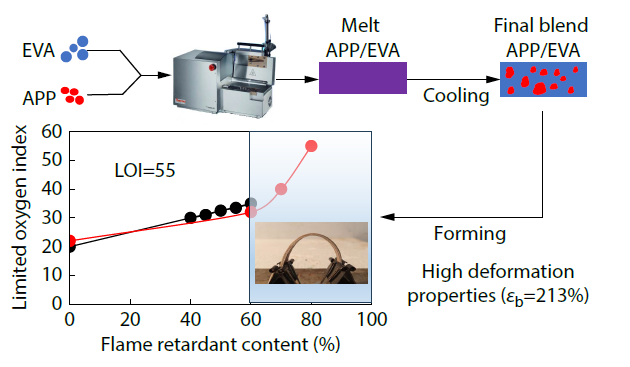
0
